Sep 26, 2022

New Technology Add-On Payments (NTAP) for 2023
In the previous three parts of this four-part series, we discussed the new ICD-10-CM diagnosis code changes, ICD-10-PCS procedure code changes and FY2023 IPPS changes. In this last Part 4 of the series, we will review the NTAP procedure codes and reimbursement add-on payments for 2023. NCTAP for Covid-19 is at the end.
Section “X” New Technology
CMS provides incremental payment (in addition to the DRG payment) for medical or new technologies that qualify for NTAP. FY2023 Medicare will make an add-on payment equal to the lesser of: (1) 75 percent medical product (65 % for new technology) of the costs of the new medical service or technology; or (2) 75 percent of the amount by which the costs of the case exceed the standard DRG payment.
CMS also finalized to extend New Technology Add-on Payments for 15 technologies from FY 2022. CMS finalized to extend New COVID-19 Treatments Add-on Payment (NCTAP) for eligible products through the end of the fiscal year in which the COVID-19 PHE ends. Keep an eye out for notifications as to when the PHE will end and affect payments.
Section “X” is a separate place within ICD-10-PCS for certain new technology procedures (such as new technology drugs). Section “X” does not introduce any new coding concepts or unusual guidelines for correct coding and maintains continuity with the other sections in ICD-10-PCS. The same root operation and body part values are used in section “X” as in other sections. The seventh characterin section “X” is used to indicate the new technology group. This is a number that changes each yearthat new technology codes are added. It is only used to indicate the year the code was created and all codes for that update year will have the same qualifier. The new technology drugs for FY2023 will have the qualifier/seventh character of “8” since this is the eighth year of ICD-10-PCS. You will see other new technology codes with numbers from previous years that are still being paid.
Section “X” codes are standalone codes. No additional codes from other sections in ICD-10-PCS are necessary for reporting as the specific procedure is described in the code title from section “X”.
New Technology section codes are easily found by looking in the ICD-10-PCS Index or the Tables. The name of the new technology device, substance or technology for a section “X” code is included as the main term. They are also listed under the main term “New Technology”.
Some new technology items have a new technology payment that is made in addition to the DRG payment for hospital inpatients. Missing new technology codes is a frequent error made by coders. Coders must familiarize themselves with the new technologies of each year and be sure to report codes for these new technologies.
New Technology Procedure Codes and Reimbursements for FY2023
Below are the codes that will be reimbursed as NTAP codes for FY2023. You may want to print this out and keep it handy or on your desktop for future reference. Some of the add on payments are astounding!
Continued from Last Year:
- VEKLURY® (remdesivir) VEKLURY® is a nucleotide analog that inhibits viral RNA-dependent RNA polymerases, demonstrating activity countering viral pathogens such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). Approved for patients 12 years or older. Hospital will get $2,028.00 as a new technology add on payment FY2023.
Codes to report:
XW033E5 - (Introduction of remdesivir anti-infective into peripheral vein, percutaneous approach, new technology group 5) or
XW043E5- (Introduction of remdesivir anti-infective into central vein, percutaneous approach, new technology group 5).
- Caption Guidance ™ Caption Guidance™ is an artificial intelligence (AI) guided medical imaging acquisition software system indicated for the acquisition of cardiac ultrasound images. The applicant explained that the system provides real-time guidance during transthoracic echocardiography (2D-TTE) to assist in obtaining anatomically correct and optimized images that represent standard 2D echocardiographic diagnostic views and orientations. The maximum payment a hospital can receive for this is $1,868.10 as a new technology add on payment Continued.
Codes to report:
X2JAX47 (Inspection of heart using transthoracic echocardiography, computer-aided guidance, new technology group 7).
- ABECMA® (idecabtagene vicleucel) Idecabtagene viclecuel is a, B-cell maturation antigen (BCMA)-directed genetically modified autologous chimeric antigen receptor (CAR) T-cell immunotherapy for the treatment of adult patients with relapsed or refractory (RR) multiple myeloma (MM) (RRMM) who have received at least four prior therapies including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 antibody (for example, triple-class-exposed). Idecabtagene vicleucel is expected to be a 5th line plus (5L+) treatment. Codes C90.00 and C90.02 for diagnoses. The maximum payment a hospital can receive for this is $ $289,532.75 as a new technology add on payment Continued.
Codes to report:
Codes C90.00 and C90.02 for diagnoses WITH:
XW033K7 - Introduction of Idecabtagene Vicleucel Immunotherapy into peripheral vein, percutaneous, new technology group 7 or
XW043K7 - Introduction of Idecabtagene Vicleucel Immunotherapy into central vein, percutaneous, new technology group 7
- ZEPZELCA™ (lurbinectedin) ZEPZELCA™ is an alkylating drug indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy. ZEPZELCA™ is a marine derived, synthetic antineoplastic compound that inhibits transcription-dependent replication stress and genome instability in tumor cells. Hospital will get $9,145.50 as a new technology add on payment Continued.
Codes to report: Code C34 for malignancy of bronchus and lung tor Z51.11/Z51.12 for admit for chemo or immunotherapy WITH:
XW03387- Introduction of Lurbinectedin percutaneous into peripheral vein New Technology 7 or
XW04387- Introduction of Lurbinectedin percutaneous into central vein New Technology 7
- COSELA (trilaciclib) COSELA (trilaciclib) is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC). Trilaciclib is a selective, transient inhibitor of cyclin dependent kinases 4 and 6 (CDK4/6) with chemoprotective activities. The CDK4/6 enzyme pathway is a key regulator of the cell cycle.1. COSELA is for intravenous use only given 30 minutes before chemothreapy. The maximum payment a hospital can receive for this is $5,612.20 as a new technology add on payment FY2023. Continued.
Codes to report:
Diagnosis code C34.- for Malignancy of bronchus or lung sites WITH:
XW03377 - Introduction of Trilaciclib into peripheral vein, percutaneous, new technology group 7 or
XW04377 - Introduction of Trilaciclib into central vein, percutaneous, new technology group 7
- RYBREVANT™ (Amivantamab) Amivantamab is intended for the treatment of metastatic non-small cell lung cancer (NSCLC). The applicant stated amivantamab is a bispecific monoclonal antibody able to inhibit the epidermal growth factor receptor (EGFR) and c-MET tyrosine kinase signaling pathways known to be involved in the pathogenesis of NSCLC. The maximum payment a hospital can receive for this is $6,405.89 as a new technology add on payment FY2023.
Codes to report:
Diagnosis code from category C34.- Carcinoma of lung and bronchus areas WITH:
XW033B7 - Introduction of Amivantamab Monoclonal Antibody into peripheral vein, percutaneous, new technology group 7 or
XW043B7 - Introduction of Amivantamab Monoclonal Antibody into central vein, percutaneous, new technology group 7
- StrataGraftTM Skin Tissue It is described as a viable, bioengineered, regenerative skin construct (BRSC) consisting of an epidermal layer of viable, fully stratified, allogeneic human NIKS®603 keratinocytes growing on a dermal layer composed of viable human dermal fibroblasts embedded in a collagen-rich matrix. The applicant noted that StrataGraftTM is intended for the treatment of adult patients with severe thermal burns that contain intact dermal elements and require surgical intervention (hereinafter referred to as severe thermal burns [STB]). Hospital will get $44,200.00 as a new technology add on payment FY2023.
Code to report:
XHRPXF7- Replacement of skin, external, Bioengineered Allogenic Construct, New Technology 7
- Tecartus™ (brexucabtagene autoleucel) Tecartus is a CD19 directed genetically modified autologous T-cell immunotherapy for the treatment of adult patients with relapsed and refractory (r/r) mantle cell lymphoma (MCL) which is a rare and aggressive subtype of non-Hodgkin Lymphoma. T cells are collected from patient then processed via leukapheresis and takes about 2-4 hours. This is then infused into patient. For autologous use only. Hospital will get $259,350.00 as a new technology add on payment FY2023.
Codes to report:
Code for dx C83.1- Mantle cell lymphoma WITH:
XW033M7- Introduction of Brexucabtagene Autoleucel Immunotherapy percutaneous into peripheral vein New Technology 7 or
XW043M7- Introduction of Brexucabtagene Autoleucel Immunotherapy percutaneous into central vein New Technology 7
- Aprevo™ Aprevo™ Intervertebral Body Fusion device. The device is an interbody fusion implant that stabilizes the lumbar spinal column and facilitates fusion during lumbar fusion procedures indicated for the treatment of spinal deformity. The applicant states that the implant device is custom made for patient-specific features, by using patient CT scans to create 3D virtual models of the deformity. Made of Titanium Alloy. The device is used during anterior lumbar interbody fusion, lateral lumbar interbody fusion, transforaminal lumbar interbody fusion, or standalone anterior lumbar interbody fusion procedures. The maximum payment a hospital can receive for this is $40,950.00 as a new technology add on payment FY2022.
Code to report:
XRG--R7, Fusion of (insert site) vertebral joint using customizable interbody fusion device, percutaneous/open approach, new technology group 7.
- aScope™ Duodeno The device is a sterile, single-use endoscope for endoscopy and endoscopic surgery indicated for treatment of the upper gastrointestinal (GI) tract. The device includes a flexible insertion tube with a bendable tip equipped with lighting and camera. aScope™ Duodeno are the types. The maximum payment a hospital can receive for this is $1,296.75 as a new technology add on payment FY2023. (Exalt Model D was discontinued)
Codes to report:
XFJB8A7 (Inspection of hepatobiliary duct using single-use duodenoscope, new technology group 7) or
XFJD8A7 (Inspection of pancreatic duct using single-use duodenoscope, new technology group 7)
- Harmony™ Transcatheter Pulmonary Valve (TPV) System The system consists of a bioprosthetic heart valve developed from porcine pericardial tissue mounted on self-expanding nitinol struts sewn to a polyester fabric. According to the applicant, Harmony™ is implanted in the patient’s heart between the right ventricle and the bifurcation of the pulmonary arteries to treat patients with congenital heart disease who are indicated for a pulmonary valve replacement. The applicant states that Harmony™ is the first transcatheter pulmonary valve that is designed to treat the patient’s condition at the native site of the pulmonary valve without a pre-existing valve conduit or pre-existing bioprosthetic valve. The maximum payment a hospital can receive for this is $26,975.00 as a new technology add on payment Continued.
Code to report:
02RH38M (Replacement of pulmonary valve with zooplastic tissue, native site, percutaneous approach).
- INTERCEPT Fibrinogen Complex (PRCFC) INTERCEPT Fibrinogen Complex is a blood product indicated for the treatment for fibrinogen deficiency-related bleeding, including massive hemorrhage. Per the applicant, this blood product is useful in emergency departments and operating rooms due to its 5-day shelf life at room temperature. The applicant stated that the 5-day shelf life of the blood product makes it immediately available in a ready-to-transfuse form as a fibrinogen source and thereby provides a significant benefit for patients with massive hemorrhage in a real time-critical fashion that is not achievable with other existing fibrinogen replacement products. The maximum payment a hospital can receive for this is $2,535.00 as a new technology add on payment Continued.
Codes to report:
Diagnosis: D62 (Acute posthemorrhagic anemia) or D65 (Disseminated intravascular coagulation) or D68.2, (Hereditary deficiency of other clotting factors) or D68.4 (Acquired coagulation factor deficiency) WITH:
30233D1 (Transfusion of nonautologous pathogen reduced cryoprecipitated fibrinogen complex into peripheral vein percutaneous approach) or
30243D1 (Transfusion of nonautologous pathogen reduced cryoprecipitated fibrinogen complex into central vein, percutaneous approach).
- Shockwave C2 Intravascular Lithotripsy (IVL) System The IVL Catheter is intended for lithotripsy-enabled, low-pressure dilation of calcified, stenotic de novo coronary arteries prior to stenting. The applicant explained that the device is delivered through the coronary arterial system, and it generates intermittent sonic waves within the target treatment site that disrupt calcium within the lesion, allowing subsequent dilation of a coronary artery stenosis using low balloon pressure. The applicant also noted that the procedure can be used for otherwise difficult to treat calcified stenosis, including calcified stenosis that are anticipated to exhibit resistance to full balloon dilation or subsequent uniform coronary stent expansion. The maximum payment a hospital can receive for this is $3,666.00 as a new technology add on payment Continued.
Codes to report:
02F 0-3 3ZZ, Fragmentation in coronary artery (one, two, three four or more) arteries,
percutaneous approach ).
- FETROJA® (A - Cefiderocol Anti-infective) FETROJA® is an injectable siderophore cephalosporin indicated for the treatment of hospital-acquired bacterial pneumonia (HABP)/ventilator-associated bacterial pneumonia (VABP) on September 25, 2020. (Different than previous slide where it was approved or UTI) Per the applicant, FETROJA® should be used to treat infections where limited or no alternative treatment options are available and where FETROJA® (cefiderocol) is likely to be an appropriate treatment option, which may include use in patients with infections caused by documented or highly suspected carbapenem-resistant and/or multidrug-resistant gram-negative (GN) pathogens. The applicant asserts that the principal antibacterial / bactericidal activity of FETROJA® occurs with inhibiting GN bacterial cell wall synthesis by binding to penicillin-binding proteins. The maximum payment a hospital can receive for this is $8,579.84 when used for HABP/VABP as a new technology add on payment FY2023. Continued as this was for new diagnosis treatment for pneumonias and not the UTI on previous approval for this same drug.
Codes to report: ICD-10-CM code Y95 and one of the following: J14, J15.0, J15.1, J15.5, J15.6, J15.8,
OR
J95.851 and one of the following:
B96.1, B96.20, B96.21, B96.22, B96.23, B96.29, B96.3, B96.5, or B96.89
WITH:
XW033A6 Introduce Cefiderocol in Peripheral Vein, Percutaneous, New Tech 6. or
XW043A6 Introduce Cefiderocol in Central Vein, Percutaneous, New Tech 6.
- RECARBRIO™ (imipenem, cilastatin, and relebactam) RECARBRIO™ is a fixed-dose combination of imipenem, a penem antibacterial; cilastatin, a renal dehydropeptidase inhibitor; and relebactam, a novel blactamase inhibitor (BLI) administered via intravenous infusion. Per the applicant, RECARBRIO™ is indicated for the treatment of hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by susceptible Gram-negative bacteria. RECARBRIO™ is also indicated for complicated urinary tract infections (cUTI) and complicated intra-abdominal infections (cIAI) and is no longer approved for NTAP). The maximum payment a hospital can receive for this is $9,576.51 as a new technology add on payment FY2023.
Codes to report: : ICD-10-CM code Y95 and one of the following: J14, J15.0, J15.1, J15.5, J15.6, J15.8,
OR
J95.851 and one of the following:
B96.1, B96.20, B96.21, B96.22, B96.23, B96.29, B96.3, B96.5, or B96.89
WITH:
XW033U5 - Introduction of Imipenem-cilastatin-relebactam Anti-infective into peripheral vein, percutaneous, new technology group 5 or
XW043U5 - Introduction of Imipenem-cilastatin-relebactam Anti-infective into central vein, percutaneous, new technology group 5
Newly Approved Traditional Pathway for 2023:
- CARVYKTI™ (Ciltacabtagene autoleucel) is an autologous chimeric-antigen receptor (CAR) T-cell therapy directed against B cell maturation antigen (BCMA) for the treatment of patients with multiple myeloma. The maximum payment a hospital can receive for this is $ $289,532.75 as a new technology add on payment Please note that this therapy is very similar to ABECMA®
Codes to report:
Codes C90.00 and C90.02 for diagnoses WITH:
XW033A7 - Introduction of Ciltacabtagene autoleucel into peripheral vein, percutaneous, new technology group 7 or
XW043A7 - Introduction of Ciltacabtagene autoleucel into central vein, percutaneous, new technology group 7
- DARZALEX FASPRO® (daratumumab and hyaluronidase-fihj) indicated for the treatment of light chain (AL) amyloidosis (E85.81) daratumumab (a monoclonal CD38-directed cytolytic antibody), and hyaluronidase (an endoglycosidase)in combination with bortezomib, cyclophosphamide and dexamethasone (CyBorD) in newly diagnosed patients and is administered through a subcutaneous injection. The maximum payment a hospital can receive for this is $ $5,159.41 as a new technology add on payment
Codes to report:
E85.81 combined WITH:
XW01318 (Introduction of daratumumab and hyaluronidase-fihj into subcutaneous tissue,
percutaneous approach, new technology group 8).
- Hemolung Respiratory Assist System (Hemolung RAS) is the first and only FDA authorized technology for the treatment of acute, hypercapnic respiratory failure using an extracorporeal circuit to remove CO2 directly from the blood. Per the applicant, patients experiencing acute, hypercapnic respiratory failure are unable to remove excess CO2 waste molecules from their blood via their lungs, resulting in accumulation of CO2 in their blood (hypercapnia), acid/base derangement (respiratory acidosis), and life-threatening clinical sequelae. The maximum payment a hospital can receive for this is $ $6,500.00 as a new technology add on payment
Code to report:
5A0920Z (Assistance with respiratory filtration, continuous
- LIVTENCITY™ (maribavir) is a cytomegalovirus (CMV) pUL97 kinase inhibitor indicated for the treatment of adults and pediatrics (12 years of age and older and weighing at least 35 kg) with post-transplant CMV infection/disease that is refractory to treatment (with or without genotypic resistance) to ganciclovir, valganciclovir, cidofovir, or foscarnet. LIVTENCITY™ is co-administered with carbamazepine, then the dosage is increased to 800 mg twice daily; if co-administered with phenytoin or phenobarbital, the dosage is increased to 1,200 mg twice daily. The maximum payment a hospital can receive for this is $32,500.00 as a new technology add on payment
Code to report:
XW0DX38 (Introduction of maribavir anti-infective into mouth and pharynx, external approach, new technology group 8), or
XW0G738 (Introduction of maribavir anti-infective into upper gi, via natural or artificial opening, new technology group 8), or
XW0H738 (Introduction of maribavir anti-infective into lower gi, via natural or artificial opening, new technology group 8).
Approved Under Alternative Pathway for 2023:
- CERAMENT® G is an injectable bone-void filler made of calcium sulfate, hydroxyapatite, and gentamicin sulfate indicated for the surgical treatment of osteomyelitis. Per the applicant, this bone graft substitute fills gaps resulting from debridement of infected bone and prevents colonization of sensitive bacteria, promoting bone healing in two ways. The maximum payment a hospital can receive for this is $4,918.55 as a new technology add on payment
Code to report:
XW0V0P7 (Introduction of antibiotic-eluting bone void filler into bones, open approach, new technology group 7).
- GORE® TAG® Thoracic Branch Endoprosthesis (TBE device) is a modular device consisting of three components, an Aortic Component, a Side Branch Component, and an optional Aortic Extender Component, each of which is pre-mounted on a catheter delivery system for treatment of thoracic aortic aneurysms, traumatic aortic transection, and aortic dissection. The maximum payment a hospital can receive for this is $27,807.00 as a new technology add on payment
Code to report:
02VW3DZ (Restriction of thoracic aorta, descending with intraluminal device, percutaneous approach), in combination WITH
02VX3EZ (Restriction of thoracic aorta, ascending/arch with branched or fenestrated intraluminal device, one or two arteries, percutaneous approach).
Both codes are needed as the device straddles the two areas.
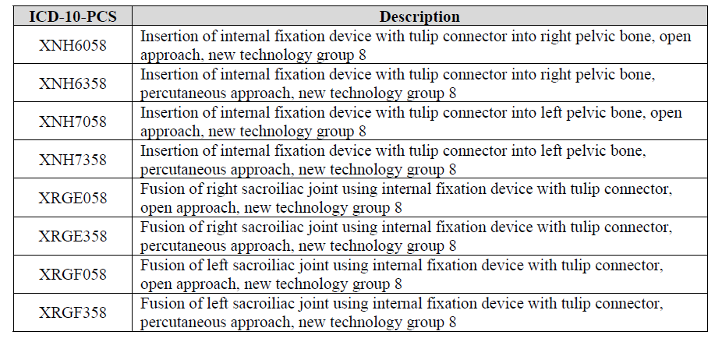
- iFuse Bedrock Granite Implant System is a sterile, single-use permanent implant intended to provide sacropelvic fusion of the sacroiliac joint and fixation to the pelvis when used in conjunction with commercially available pedicle screw fixation systems as a foundational element for segmental spinal fusion. The applicant states that the joint fusion occurs as a result of the device’s porous surface and interstices, and fixation occurs through the device’s helical threaded design and traditional posterior fixation rod connection. The maximum payment a hospital can receive for this is $9,828.00 as a new technology add on payment.
Code to report: One from this list of new codes for the device for FY2023:
- Thoraflex™ Hybrid Device is a sterile single-use, gelatin sealed Frozen Elephant Trunk (FET) surgical medical device. (Prevents blood loss and acts as a seal). For diseased or damaged aortic arch, descending and possibly ascending aorta. The device is deployed through an opened aortic arch and then positioned into the descending thoracic aorta. Once it is completely deployed, the collar is sutured to the aorta, and graft anastomoses are then performed in a manner depending upon the chosen product design (which the applicant specified as either the Plexus or the Ante-Flo). The device includes a proximal crimped polyester surgical graft, central polyester collar, and distal nitinol ring stents supported by thin wall polyester fabric. The maximum payment a hospital can receive for this is $22,750.00 as a new technology add on payment
Codes to report:
X2RX0N7 (Replacement of thoracic aorta arch with branched synthetic substitute with intraluminal device, new technology group 7)
In Combination WITH
X2VW0N7 (Restriction of thoracic descending aorta with branched synthetic substitute with intraluminal device, new technology group 7)
Both codes must be reported!
- ViviStim® Paired VNS System is a paired vagus nerve stimulation therapy intended to stimulate the vagus nerve during rehabilitation therapy to reduce upper extremity motor deficits and improve motor function in chronic ischemic stroke patients with moderate to severe arm impairment. The Vivistim® Paired VNS System is comprised of an Implantable Pulse Generator (IPG), an implantable stimulation Lead, and an external paired stimulation controller which is composed of the external Wireless Transmitter (WT) and the external Stroke Application and Programming Software (SAPS). The maximum payment a hospital can receive for this is $23,400.00 as a new technology add on payment
Code to report:
X0HQ3R8 (Insertion of neurostimulator lead with paired stimulation system into vagus nerve, percutaneous approach, new technology group 8).
- DefenCath™ (solution of taurolidine (13.5 mg/mL) and heparin (1000 USP Units/mL)) is a proprietary formulation of taurolidine, a thiadiazinane antimicrobial, and heparin, an anti-coagulant, which is under development for use as catheter lock solution, with the aim of reducing the risk of catheter-related bloodstream infections (CRBI) from in-dwelling catheters in patients undergoing hemodialysis (HD) through a central venous catheter (CVC). According to the applicant, in vitro studies of DefenCath™ indicate broad antimicrobial activity against gram-positive and gram-negative bacteria, including antibiotic resistant strains as well as mycobacteria and clinically relevant fungi). The maximum payment a hospital can receive for this is $4,387.50 as a new technology add on payment
Code to report:
XY0YX28 (Extracorporeal introduction of taurolidine anti-infective and heparin anticoagulant, new technology group 8).
New COVID-19 Treatments Add-On Payment (NCTAP)
https://www.cms.gov/medicare/covid-19/new-covid-19-treatments-add-payment-nctap
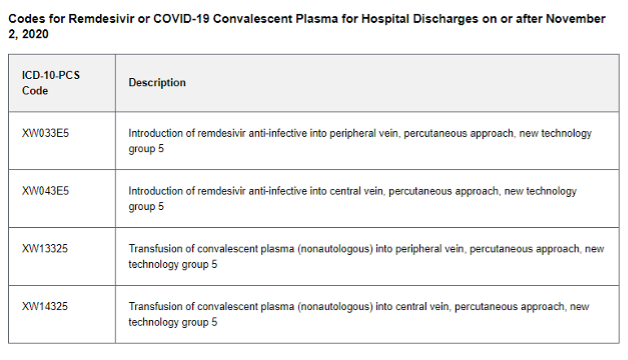
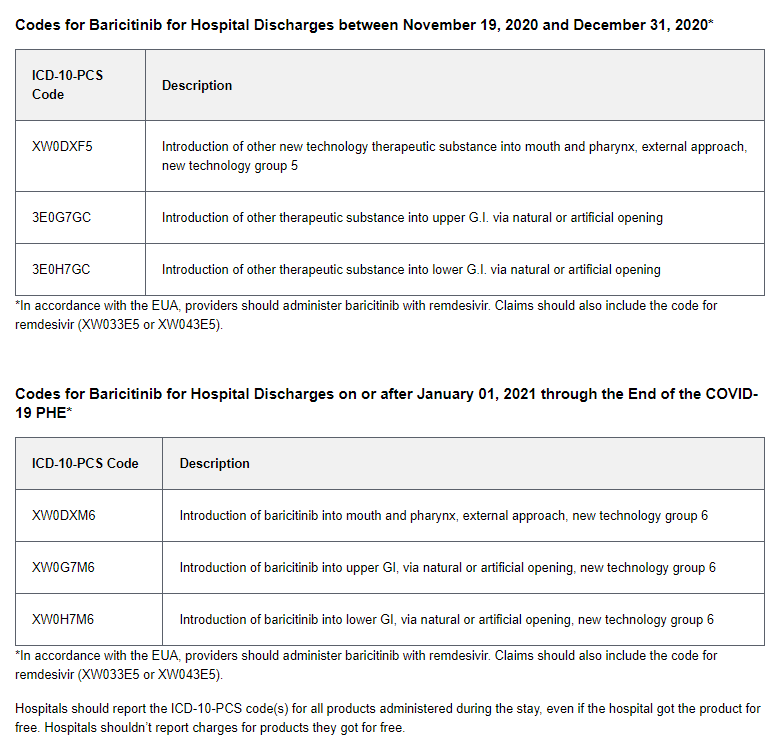
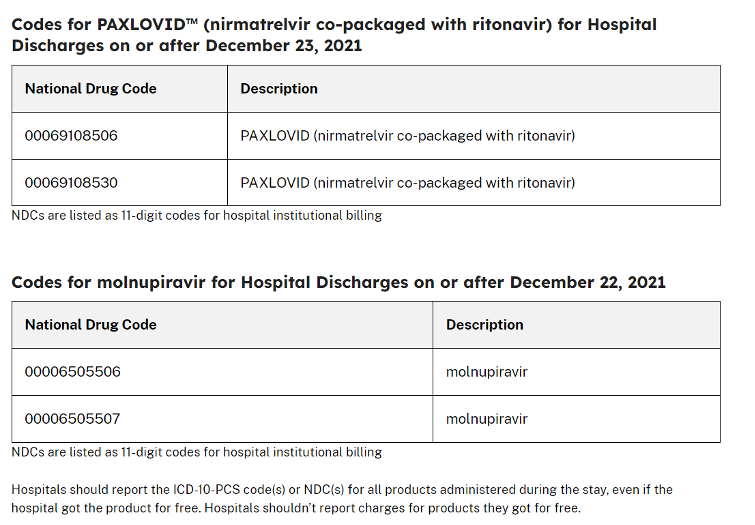
NCTAP claims are those that are eligible for the 20% add-on payment under Section 3710 of the CARES Act. Eligible claims have an ICD-10-CM diagnosis code U07.1 (COVID-19) and one of the following:
ICD-10-PCS codes for remdesivir (Veklury), COVID-19 convalescent plasma, or baricitinib (Olumiant)
National Drug Codes (NDC) for PAXLOVID (nirmatrelvir co-packaged with ritonavir) and molnupiravir
Subscribe to our Newsletter
Recent Blogs
Related blogs from Industry News , Medical Coding Tips
CMS Hospital Compare is a public reporting pl...
Premier benchmarking is widely used by hospit...
PEPPER reports help U.S. hospitals identify o...
CMS has released the updates to the ICD-10-PC...
Subscribe
to our Newsletter
Weekly medical coding tips and coding education delivered directly to your inbox.




