In the previous three parts of this four-part series, we discussed the new ICD-10-CM diagnosis code changes, ICD-10-PCS procedure code changes and FY2022 IPPS changes. In this last Part 4 of the series, we will review the NTAP procedure codes and reimbursement add-on payments for FY2022. Prepare yourself as this is rather lengthy due to continuation of NTAP that would normally expire.
Section “X” New Technology
CMS provides incremental payment (in addition to the DRG payment) for medical or new technologies that qualify for NTAP. FY2022 Medicare will make an add-on payment equal to the lesser of: (1) 75 percent medical product (65 % for new technology) of the costs of the new medical service or technology; or (2) 75 percent of the amount by which the costs of the case exceed the standard DRG payment.
CMS also finalized to extend New Technology Add-on Payments for 14 technologies that otherwise would be discontinued in FY 2022. CMS finalized to extend New COVID-19 Treatments Add-on Payment (NCTAP) for eligible products through the end of the fiscal year in which the COVID-19 PHE ends. CMS is also finalizing to discontinue NCTAP for discharges on or after Oct. 1, 2021 for a product that is approved for new-technology add-on payments beginning in FY 2022.
Section “X” is a separate place within ICD-10-PCS for certain new technology procedures (such as new technology drugs). Section “X” does not introduce any new coding concepts or unusual guidelines for correct coding and maintains continuity with the other sections in ICD-10-PCS. The same root operation and body part values are used in section “X” as in other sections. The seventh character in section “X” is used to indicate the new technology group. This is a number that changes each year that new technology codes are added. It is only used to indicate the year the code was created and all codes for that update year will have the same qualifier. The new technology drugs for FY2022 will have the qualifier/seventh character of “7” since this is the seventh year of ICD-10-PCS. You will see other new technology codes with numbers from previous years that are still being paid.
Section “X” codes are standalone codes. No additional codes from other sections in ICD-10-PCS are necessary for reporting as the specific procedure is described in the code title from section “X”.
New Technology section codes are easily found by looking in the ICD-10-PCS Index or the Tables. The name of the new technology device, substance or technology for a section “X” code is included as the main term. They are also listed under the main term “New Technology”.
Some new technology items have a new technology payment that is made in addition to the DRG payment for hospital inpatients. Missing new technology codes is a frequent error made by coders. Coders must familiarize themselves with the new technologies of each year and be sure to report codes for these new technologies.
New Technology Procedure Codes and Reimbursements for FY2022
Below are the codes that will be reimbursed as NTAP codes. There are 16 of 26 approved for NTAP for FY2022. You may want to print this out and keep it handy or on your desktop for future reference. Some of the add on payments are astounding!
Continued approval from last year:
ZEMDRI™(Plazonmicin). Next generation aminoglycoside antibiotic to treat multi-drug resistant gram-negative bacteria. Usually complicated UTI (cUTI) or pyelonephritis. The maximum payment a hospital can receive for this is $4,083.75 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW033G4 – Introduction of Plazomicin Anti-Infective into peripheral vein, percutaneous, new technology group 4 or
XW043G4 – Introduction of Plazomicin Anti-Infective into central vein, percutaneous, new technology group 4
AndexXa™ (Andexanet alfa) (coagulation factor Xa (recombinant), inactivated-zhzo) is an antidote used to treat patients who are receiving treatment with an oral Factor Xa inhibitor who suffer a major bleeding episode and require urgent reversal of direct and indirect Factor Xa anticoagulation. Patients at high risk for thrombosis, including those who have been diagnosed with atrial fibrillation (AF) and venous thrombosis (VTE), typically receive treatment using long-term oral anticoagulation agents. The maximum payment a hospital can receive for this is $18,281.25 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW03372 – Introduction of Andexanet Alfa, Factor Xa Inhibitor Reversal agent into peripheral vein, percutaneous, new technology group 2 or
XW04372 – Introduction of Andexanet Alfa, Factor Xa Inhibitor Reversal agent into central vein, percutaneous, new technology group 2
AZEDRA® (Ultratrace® iobenguane Iodine-131) is a drug solution for IV use in patients with obenguane avid malignant and/or recurrent and/or unresectable pheochroomocytoma and paraganglioma (PPGL). These are rare with incidence of 2-8 people per million per year. Paragangliomas have a malignancy frequency of 25%. They are both neuroendocrine tumors. The maximum payment a hospital can receive for this is $98,150 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW033S5 – Introduction of Iobenguane I-131 Antineoplastic into peripheral vein, percutaneous, new technology group 5 or
XW043S5 – – Introduction of Iobenguane I-131 Antineoplastic into central vein, percutaneous, new technology group 5
CABLIVI® (caplacizumab-yhdp) is a humanized bivalent nanobody administered through IV and subcutaneous injections to inhibit micro clot formation in adult patients how have been diagnosed with thrombotic thrombocytopenic purpura (aTTP). The maximum payment a hospital can receive for this is $33,215 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW013W5 – Introduction of Caplacizumab into subcutaneous tissue, percutaneous, new technology group 5 or
XW033W5 – Introduction of Caplacizumab into peripheral vein, percutaneous, new technology group 5 or
XW043W5 – Introduction of Caplacizumab into central vein, percutaneous, new technology group 5
ELZONRIS™ (tagraxofusp-erzs) is a targeted therapy for treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) which is highly aggressive. Administered via infusion. The maximum payment a hospital can receive for this is $144,116.04 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW033Q5 – Introduction of Tagraxofusp-erzs Antineoplastic into peripheral vein, percutaneous, new technology group 5 or
XW043Q5 – Introduction of Tagraxofusp-erzs Antineoplastic into central vein, percutaneous, new technology group 5
Balversa™ (Erdafitimib) is an oral medication indicated for the second-line treatment of adult patients who have been diagnosed with locally advanced or metastatic urothelial carcinoma whose tumors exhibit certain fibroblast growth factor receptor (FGFR) genetic alterations as detected by an FDA-approved test, and who have disease progression during or following at least one line of prior chemotherapy including within 12 months of neoadjuvant or adjuvant chemotherapy. The maximum payment a hospital can receive for this is $3,563.23 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 4/12/22)
- Code to report:
XW0DXL5 – Introduction of Erdafitinib Antineoplastic into mouth and pharynx, external, new technology group 5
SPRAVATO (Esketamine) is a nasal spray used for treatment-resistant (major) depression (TRD). The maximum payment a hospital can receive for this is $1,014.79 as a new technology add on payment. (Given a one-year extension)
- Code to report:
XW097M5 – Introduce Esketamine Hcl in Nose, Via Opening, New Tech 5
XOSPATA® (gilteritinib) is an oral medication indicated for the treatment of adult patients who have been diagnosed with relapsed or refractory acute myeloid leukemia (AML) with an FMS-like tyrosine kinase 3 (FLT3) mutation as detected by an FDA-approved test. The maximum payment a hospital can receive for this is $7,312.50 as a new technology add on payment. (Given a one-year extension)
- Code to report:
XW0DXV5 – Introduction of Gilteritinib Antineoplastic into mouth and pharynx, external approach, new technology group 5
JAKAFI™ (Ruxolitinib) is an oral kinase inhibitor that inhibits Janus-associated kinases 1 and 2 (JAK1/JAK2). The JAK pathway, which includes JAK1 and JAK2, is involved in the regulation of immune cell maturation and function. According to the applicant, JAK inhibition represents a novel therapeutic approach for the treatment of acute graft-versus-host disease (GVHD) in patients who have had an inadequate response to corticosteroids. The maximum payment a hospital can receive for this is $4,475.38 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 5/24/22)
- Code to report:
XW0DXT5 – Introduction of Ruxolitinib into mouth and pharynx, external approach, new technology group 5
T2Bacteria® Panel (T2 Bacteria Test Panel) Is a lab test indicated as an aid in the diagnosis of bacteremia, bacterial presence in the blood which is a precursor for sepsis. The maximum payment a hospital can receive for this is $97.50 as a new technology add on payment. (Given a one-year extension)
- Code to report:
XXE5XM5 – Measurement, circulatory, external, infection, whole blood nucleic acid-base microbial detection, new technology group 5
ContaCT (“Viz LVO”, “Viz View”) is a radiological computer-assisted triage and notification software system intended for use by hospital networks and trained clinicians. The applicant asserted that ContaCT analyzes computed tomography angiogram (CTA) images of the brain acquired in the acute setting, sends notifications to a neurovascular specialist(s) that a suspected large vessel occlusion (LVO) has been identified, and recommends review of those images. Helps to reduce the time to treat stoke patients. The maximum payment a hospital can receive for this is $1,040.00 as a new technology add on payment. (Given a one-year extension)
- Code to report:
4A03X5D – Measurement, arterial flow, external, intracranial
Eluvia™ Drug-Eluting Vascular Stent System (Eluvia) Is a sustained released drug-eluting stent for the treatment of lesions in the femoropopliteal arteries. The maximum payment a hospital can receive for this is $3,646.50 as a new technology add on payment. (Given a one-year extension)
- Codes to report: 16 Dilation codes
X27—5 – Dilation, various femoropopliteal arteries (select artery), percutaneous approach, various number of sustained release drug-eluting intraluminal devices (choose number) new technology group 5
Hemospray® Endoscopic Hemostat is indicated by the FDA for hemostasis of nonvariceal gastrointestinal bleeding. Using an endoscope to access the gastrointestinal tract, the Hemospray delivery system is passed through the accessory channel of the endoscope and positioned just above the bleeding site without making contact with the GI tract wall. The Hemospray powder, bentonite, is propelled through the application catheter, either a 7 or 10 French polyethylene catheter, by release of CO2 from the cartridge located in the device handle and sprayed onto the bleeding site. The maximum payment a hospital can receive for this is $1,625.00 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW0G886 – Introduction of mineral-based topical hemostatic agent into upper GI, via natural or artificial opening, endoscopic, new technology group 6 or
XW0H886 – Introduction of mineral-based topical hemostatic agent into lower GI, via natural or artificial opening, endoscopic, new technology group 6
IMFINZI® (durvalumab) (AstraZeneca PLS) and TECENTRIQ® (atezolizumab) are indicated in treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum containing chemotherapy. The maximum payment a hospital can receive for this is $6,875.90 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW03336 – Introduction of Durvalumab Antineoplastic into peripheral vein, percutaneous, new technology group 6 or
XW04336 – Introduction of Durvalumab Antineoplastic into central vein, percutaneous, new technology group 6 or
XW033D6 – Introduction of Atezolizumab Antineoplastic into peripheral vein, percutaneous, new technology group 6 or
XW043D6 – Introduction of Atezolizumab Antineoplastic into central vein, percutaneous, new technology group 6With a diagnosis code from category C34.- with Z51.11 or Z51.12 Encounter for chemotherapy/immunotherapy
Soliris ® is approved for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adult patients who are anti-aquaporin-4 (AQP4) antibody positive. The maximum payment a hospital can receive for this is $21,199.75 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 6/27/22)
- Codes to report:
XW033C6 – Introduction of Eculizumab into peripheral vein, percutaneous, new technology group 6 or
XW043C6 – Introduction of Eculizumab into central vein, percutaneous, new technology group 6
The SpineJack® System is an implantable fracture reduction system, which is indicated for use in the reduction of painful osteoporotic vertebral compression fractures (VCFs) and is intended to be used in combination with Stryker VertaPlex and VertaPlex High Viscosity (HV) bone cement. Implanted into a collapsed vertebral body (VB) via a percutaneous transpedicular approach under fluoroscopic guidance. Helps to reduce the time to treat stoke patients. The maximum payment a hospital can receive for this is $3,654.72 as a new technology add on payment. (Given a one-year extension)
- Code to report:
INDEX: SpineJack(R) system use Synthetic Substitute, Mechanically Expandable (Paired) in New Technology
XNU – New Technology, Bones, Supplement
XNU0356 – Lumbar Vertebra
XNU4356 – Thoracic Vertebra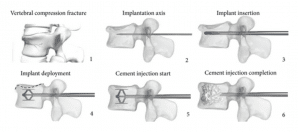
BAROSTIM NEO® System is indicated for the improvement of symptoms of heart failure – quality of life, six-minute hall walk and functional status – for patients who remain symptomatic despite treatment with guideline-directed medical therapy, are NYHA Class III or Class II (who had a recent history of Class III), have a left ventricular ejection fraction ≤ 35%, a NT-proBNP < 1600 pg/ml and excluding patients indicated for Cardiac Resynchronization Therapy (CRT) according to AHA/ACC/ESC guidelines. The maximum payment a hospital can receive for this is $ $22,750 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 8/16/22)
- Codes to report:
0JH60MZ – Insertion, stimulator generator into subcutaneous tissue and fascia, chest, open
WITH
03HK0MZ – Insertion, simulator lead into internal carotid artery, right, open
OR
03HL0MZ- Insertion, simulator lead into internal carotid artery, left, open
Optimizer System is Intended for the treatment of chronic heart failure in patients with advanced symptoms that have normal QRS duration and are not indicated for cardiac resynchronization therapy. consists of three components. First, the Optimizer Rechargeable Implantable Pulse Generator (IPG) is designed for subcutaneous implant and delivers cardiac contractility modulation to the heart via two standard pacing leads attached to the right ventricular septum. Second, the Optimizer Mini Charger recharges the Optimizer IPG. Finally, the Omni II Programmer with Omni SMART Software gives a qualified healthcare professional the ability to program the Optimizer IPG over a large range of clinical settings The maximum payment a hospital can receive for this is $14,950.00 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 10/23/22)
- Codes to report:
0JH60AZ – Insertion contractility modulation device into subcutaneous tissue and fascia, chest, open
OR
0JH63AZ- Insertion contractility modulation device into subcutaneous tissue and fascia, chest, percutaneous
OR
0JH80AZ- Insertion contractility modulation device into subcutaneous tissue and fascia, abdomen, open
OR
0JH83AZ- Insertion contractility modulation device into subcutaneous tissue and fascia, abdomen, percutaneous
AND
02HK0MZ – Insertion, cardiac lead, right ventricle, open
OR
02HK3MZ – Insertion, cardiac lead, right ventricle, percutaneous
OR
02H60MZ – Insertion, cardiac lead, right atrium, open
OR
02H63MZ- Insertion, cardiac lead, right atrium, percutaneous
Fetroja (Cefiderocol) is an injectable β-lactam antibiotic indicated for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, caused by the following susceptible Gram-negative (GN) pathogens: Escherichia coli (including with concurrent bacteremia), Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Citrobacter freundii, Enterobacter cloacae, Morganellamorganii, and Serratia marcescens. Cefiderocol should be used to treat infections where limited or no alternative treatment options are available and where cefiderocol is likely to be an appropriate treatment option, which may include use in patients with infections caused by documented or highly suspected carbapenem-resistant (CR) and/or multidrug-resistant GN pathogens. The maximum payment a hospital can receive for this is $7,919.86 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 2/24/23)
- Codes to report:
XW033A6 – Introduction of Cefiderocol Anti-infective into peripheral vein, percutaneous, new technology group 6 or
XW043A6 – Introduction of Cefiderocol Anti-infective into central vein, percutaneous, new technology group 6
NUZYRA® (omadacycline) for Injection is a tetracycline class antibacterial indicated for the treatment of adult patients with the following infections caused by susceptible microorganisms: Community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI). A single-does vial containing 100 mg. The maximum payment a hospital can receive for this is $1,552.50 as a new technology add on payment. (Given a one-year extension)
- Codes to report:
XW033B6 – Introduction of Omadacycline Anti-infective into peripheral vein, percutaneous, new technology group 6 or
XW043B6 – Introduction of Omadacycline Anti-infective into central vein, percutaneous, new technology group 6
RECARBRIO™ (imipenem, cilastatin, and relebactam) administered by intravenous infusion over 30 minutes as a fixed-dose combination of imipenem, a penem antibacterial; cilastatin, a renal dehydropeptidase inhibitor; and relebactam, a novel β-lactamase inhibitor (BLI). According to the applicant, RECARBRIO™ is intended for the treatment of complicated urinary tract infections (cUTI) and complicated intra-abdominal infections (cIAI) for patients 18 years of age and older. The maximum payment a hospital can receive for this is $3,532.78 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 1/6/23) (See below as also approved as a NEW tech for other conditions)
- Codes to report:
XW033U5 – Introduction of Imipenem-cilastatin-relebactam Anti-infective into peripheral vein, percutaneous, new technology group 5 or
XW043U5 – Introduction of Imipenem-cilastatin-relebactam Anti-infective into central vein, percutaneous, new technology group 5
XENLETA (Lefamulin) a pleuromutilin antibacterial agent representing the first intravenous (IV) and oral treatment option from a novel class of antibiotics for community-acquired bacterial pneumonia (CABP). Can be given orally or by injection. The maximum payment a hospital can receive for this is $1,275.75 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 9/10/22)
- Codes to report:
XW03366 – Introduction of Imipenem-cilastatin-relebactam Anti-infective into peripheral vein, percutaneous, new technology group 5 or
XW04366 – Introduction of Imipenem-cilastatin-relebactam Anti-infective into central vein, percutaneous, new technology group 5
XW0DX66 – Introduction of Imipenem-cilastatin-relebactam Anti-infective into central vein, percutaneous, new technology group
ZERBAXA® (ceftolozane and tazobactam) is a combination of ceftolozane, a cephalosporin antibacterial; and tazobactam, a β-lactamase inhibitor (BLI), indicated in patients 18 years or older for the treatment of the following infections caused by designated susceptible microorganisms:
- Complicated Intra-abdominal Infections (cIAI), used in combination with metronidazole;
- Complicated Urinary Tract Infections (cUTI), Including Pyelonephritis;
- Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP).
The maximum payment a hospital can receive for this is $1836.98 as a new technology add on payment. (Continued due to 3-year anniversary date occurs on 6/3/22)
- Codes to report:
XW03396 – Introduction of Ceftolozane/Tazobactam Anti-infective into peripheral vein, percutaneous, new technology group 6 or
XW04396 – Introduction of Ceftolozane/Tazobactam Anti-infective into central vein, percutaneous, new technology group 6
Newly approved for FY2022
RYBREVANT™ (Amivantamab) Amivantamab is intended for the treatment of metastatic non-small cell lung cancer (NSCLC). The applicant stated amivantamab is a bispecific monoclonal antibody able to inhibit the epidermal growth factor receptor (EGFR) and c-MET tyrosine kinase signaling pathways known to be involved in the pathogenesis of NSCLC. The maximum payment a hospital can receive for this is $6,405.89 as a new technology add on payment FY2022.
- Codes to report:
Diagnosis code from category C34.- Carcinoma of lung and bronchus areas and
XW033B7 – Introduction of Amivantamab Monoclonal Antibody into peripheral vein, percutaneous, new technology group 7 *New ICD-10-PCS code for FY2022
XW043B7 – Introduction of Amivantamab Monoclonal Antibody into central vein, percutaneous, new technology group 7 *New ICD-10-PCS code for FY2022
COSELA (trilaciclib) COSELA (trilaciclib) is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC). Trilaciclib is a selective, transient inhibitor of cyclin dependent kinases 4 and 6 (CDK4/6) with chemoprotective activities. The CDK4/6 enzyme pathway is a key regulator of the cell cycle.1. COSELA is for intravenous use only given 30 minutes before chemothreapy. The maximum payment a hospital can receive for this is $5,526.30 as a new technology add on payment FY2022.
- Codes to report:
Diagnosis code C34.- for Malignancy of bronchus or lung sites and
XW03377 – Introduction of Trilaciclib into peripheral vein, percutaneous, new technology group 7 *New ICD-10-PCS code for FY2022
XW04377 – Introduction of Trilaciclib into central vein, percutaneous, new technology group 7 *New ICD-10-PCS code for FY2022
ABECMA® (idecabtagene vicleucel) Idecabtagene viclecuel is a, B-cell maturation antigen (BCMA)-directed genetically modified autologous chimeric antigen receptor (CAR) T-cell immunotherapy for the treatment of adult patients with relapsed or refractory (RR) multiple myeloma (MM) (RRMM) who have received at least four prior therapies including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 antibody (for example, triple-class-exposed). Idecabtagene vicleucel is expected to be a 5th line plus (5L+) treatment. Codes C90.00 and C90.02 for diagnoses. The maximum payment a hospital can receive for this is $272,675.00 as a new technology add on payment FY2022.
- Codes to report:
Codes C90.00 and C90.02 for diagnoses
XW033K7 – Introduction of Idecabtagene Vicleucel Immunotherapy into peripheral vein, percutaneous, new technology group 7 *New ICD-10-PCS code for FY2022
XW043K7 – Introduction of Idecabtagene Vicleucel Immunotherapy into central vein, percutaneous, new technology group 7 *New ICD-10-PCS code for FY2022
StrataGraftTM Skin Tissue It is described as a viable, bioengineered, regenerative skin construct (BRSC) consisting of an epidermal layer of viable, fully stratified, allogeneic human NIKS®603 keratinocytes growing on a dermal layer composed of viable human dermal fibroblasts embedded in a collagen-rich matrix. The applicant noted that StrataGraftTM is intended for the treatment of adult patients with severe thermal burns that contain intact dermal elements and require surgical intervention (hereinafter referred to as severe thermal burns [STB]). Hospital will get $44,200 as a new technology add on payment FY2022.
- Code to report:
XHRPXF7- Replacement of skin, external, Bioengineered Allogenic Construct, New Technology 7 *New ICD-10-PCS code for FY2022
Tecartus™ (brexucabtagene autoleucel) Tecartus is a CD19 directed genetically modified autologous T-cell immunotherapy for the treatment of adult patients with relapsed and refractory (r/r) mantle cell lymphoma (MCL) which is a rare and aggressive subtype of non-Hodgkin Lymphoma. T cells are collected from patient then processed via leukapheresis and takes about 2-4 hours. This is then infused into patient. For autologous use only. Hospital will get $259,350 as a new technology add on payment FY2022.
- Codes to report:
Code for dx C83.1- Mantle cell lymphoma
XW033M7- Introduction of Brexucabtagene Autoleucel Immunotherapy percutaneous into peripheral vein New Technology 7 *New ICD-10-PCS code for FY2022
XW043M7- Introduction of Brexucabtagene Autoleucel Immunotherapy percutaneous into central vein New Technology 7 *New ICD-10-PCS code for FY2022
VEKLURY® (remdesivir) VEKLURY® is a nucleotide analog that inhibits viral RNA-dependent RNA polymerases, demonstrating activity countering viral pathogens such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). Approved for patients 12 years or older. Hospital will get $2,028 as a new technology add on payment FY2022.
- Codes to report:
XW033E5 – (Introduction of remdesivir anti-infective into peripheral vein, percutaneous approach, new technology group 5)
XW043E5- (Introduction of remdesivir anti-infective into central vein, percutaneous approach, new technology group 5).
ZEPZELCA™ (lurbinectedin) ZEPZELCA™ is an alkylating drug indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy. ZEPZELCA™ is a marine derived, synthetic antineoplastic compound that inhibits transcription-dependent replication stress and genome instability in tumor cells. Code C34 for malignancy of bronchus and lung tor Z51.11/Z51.12 for admit for chemo or immunotherapy. Hospital will get $8,622.90 as a new technology add on payment FY2022.
- Codes to report:
XW03387- Introduction of Lurbinectedin percutaneous into peripheral vein New Technology 7 *New ICD-10-PCS code for FY2022
XW04387- Introduction of Lurbinectedin percutaneous into central vein New Technology 7 *New ICD-10-PCS code for FY2022
Approved under alternative pathway for FY2022
Aprevo™ Aprevo™ Intervertebral Body Fusion device. The device is an interbody fusion implant that stabilizes the lumbar spinal column and facilitates fusion during lumbar fusion procedures indicated for the treatment of spinal deformity. The applicant states that the implant device is custom made for patient-specific features, by using patient CT scans to create 3D virtual models of the deformity. Made of Titanium Alloy. The device is used during anterior lumbar interbody fusion, lateral lumbar interbody fusion, transforaminal lumbar interbody fusion, or standalone anterior lumbar interbody fusion procedures. The maximum payment a hospital can receive for this is $20,475 as a new technology add on payment FY2022. orthoworld.com/carlsmed-aprevo-interbody-gains-fda-clearance-breakthrough-device-designation/ Approved.
- Codes to report:
XRG–R7, Fusion of (insert site) vertebral joint using customizable interbody fusion device, percutaneous/open approach, new technology group 7. *New ICD-10-PCS codes for FY2022
aScope™ Duodeno OR EXALT™ Model D The device is a sterile, single-use endoscope for endoscopy and endoscopic surgery indicated for treatment of the upper gastrointestinal (GI) tract. The device includes a flexible insertion tube with a bendable tip equipped with lighting and camera. aScope™ Duodeno or EXALT™ Model D are the types. The maximum payment a hospital can receive for this is $1,715.59 as a new technology add on payment FY2022. Approved.
- Codes to report:
XFJB8A7 (Inspection of hepatobiliary duct using single-use duodenoscope, new technology group 7) *New ICD-10-PCS code for FY2022
XFJD8A7 (Inspection of pancreatic duct using single-use duodenoscope, new technology group 7) *New ICD-10-PCS code for FY2022
Caption Guidance ™ Caption Guidance™ is an artificial intelligence (AI) guided medical imaging acquisition software system indicated for the acquisition of cardiac ultrasound images. The applicant explained that the system provides real-time guidance during transthoracic echocardiography (2D-TTE) to assist in obtaining anatomically correct and optimized images that represent standard 2D echocardiographic diagnostic views and orientations. The maximum payment a hospital can receive for this is $1,868.10 as a new technology add on payment FY2022. Approved.
- Codes to report:
X2JAX47 (Inspection of heart using transthoracic echocardiography, computer-aided guidance, new technology group 7). *New ICD-10-PCS code for FY2022
Harmony™ Transcatheter Pulmonary Valve (TPV) System The system consists of a bioprosthetic heart valve developed from porcine pericardial tissue mounted on self-expanding nitinol struts sewn to a polyester fabric. According to the applicant, Harmony™ is implanted in the patient’s heart between the right ventricle and the bifurcation of the pulmonary arteries to treat patients with congenital heart disease who are indicated for a pulmonary valve replacement. The applicant states that Harmony™ is the first transcatheter pulmonary valve that is designed to treat the patient’s condition at the native site of the pulmonary valve without a pre-existing valve conduit or pre-existing bioprosthetic valve. The maximum payment a hospital can receive for this is $26,975 as a new technology add on payment FY2022. Approved.
- Code to report:
02RH38M (Replacement of pulmonary valve with zooplastic tissue, native site, percutaneous approach) *New ICD-10-PCS code for FY2022
INTERCEPT Fibrinogen Complex (PRCFC) INTERCEPT Fibrinogen Complex is a blood product indicated for the treatment for fibrinogen deficiency-related bleeding, including massive hemorrhage. Per the applicant, this blood product is useful in emergency departments and operating rooms due to its 5-day shelf life at room temperature. The applicant stated that the 5-day shelf life of the blood product makes it immediately available in a ready-to-transfuse form as a fibrinogen source and thereby provides a significant benefit for patients with massive hemorrhage in a real time-critical fashion that is not achievable with other existing fibrinogen replacement products. The maximum payment a hospital can receive for this is $2,535 as a new technology add on payment FY2022. Approved.
- Codes to report:
Diagnosis: D65 (Disseminated intravascular coagulation) or D68.2, (Hereditary deficiency of other clotting factors) and
30233D1 (Transfusion of nonautologous pathogen reduced cryoprecipitated fibrinogen complex into peripheral vein percutaneous approach). *New ICD-10-PCS code for FY2022
30243D1 (Transfusion of nonautologous pathogen reduced cryoprecipitated fibrinogen complex into central vein, percutaneous approach). *New ICD-10-PCS code for FY2022
Shockwave C2 Intravascular Lithotripsy (IVL) System The IVL Catheter is intended for lithotripsy-enabled, low-pressure dilation of calcified, stenotic de novo coronary arteries prior to stenting. The applicant explained that the device is delivered through the coronary arterial system, and it generates intermittent sonic waves within the target treatment site that disrupt calcium within the lesion, allowing subsequent dilation of a coronary artery stenosis using low balloon pressure. The applicant also noted that the procedure can be used for otherwise difficult to treat calcified stenosis, including calcified stenosis that are anticipated to exhibit resistance to full balloon dilation or subsequent uniform coronary stent expansion. The maximum payment a hospital can receive for this is $3,666 as a new technology add on payment FY2022. Approved.
- Codes to report:
02F 0-3 3ZZ, Fragmentation in coronary artery (one, two, three four or more) arteries, percutaneous approach ). *New ICD-10-PCS codes for FY2022
CONTEPO™ (fosfomycin) CONTEPO™ is an intravenously administered epoxide antibiotic intended for the treatment of complicated urinary tract infections (cUTI) including acute pyelonephritis (AP) caused by designated susceptible bacteria. Per the applicant, the drug inhibits cell wall synthesis at an earlier stage and provides new treatment for patients with cUTIs including acute pyelonephritis caused by Escherichia coli and Klebsiella pneumonia that have failed to respond to other first-line therapies. The maximum payment a hospital can receive for this is $2,625 as a new technology add on payment FY2022. Approved.
- Codes to report:
XW033K5 (Introduction of fosfomycin anti-infective into peripheral vein, percutaneous approach, new technology group 5
XW043K5 (Introduction of fosfomycin anti-infective into central vein, percutaneous approach, new technology group 5
FETROJA® (A – Cefiderocol Anti-infective) FETROJA® is an injectable siderophore cephalosporin indicated for the treatment of hospital-acquired bacterial pneumonia (HABP)/ventilator-associated bacterial pneumonia (VABP) on September 25, 2020. (Different than previous slide where it was approved or UTI) Per the applicant, FETROJA® should be used to treat infections where limited or no alternative treatment options are available and where FETROJA® (cefiderocol) is likely to be an appropriate treatment option, which may include use in patients with infections caused by documented or highly suspected carbapenem-resistant and/or multidrug-resistant gram-negative (GN) pathogens. The applicant asserts that the principal antibacterial / bactericidal activity of FETROJA® occurs with inhibiting GN bacterial cell wall synthesis by binding to penicillin-binding proteins. The maximum payment a hospital can receive for this is $8,579.84 when used for HABP/VABP as a new technology add on payment FY2022. Approved as new as this was for new diagnosis treatment for pneumonias and not the UTI on previous entry above.
- Codes to report:
XW033A6 Introduce Cefiderocol in Peripheral Vein, Percutaneous, New Tech 6.
XW043A6 Introduce Cefiderocol in Central Vein, Percutaneous, New Tech 6.
RECARBRIO™ (imipenem, cilastatin, and relebactam) RECARBRIO™ is a fixed-dose combination of imipenem, a penem antibacterial; cilastatin, a renal dehydropeptidase inhibitor; and relebactam, a novel blactamase inhibitor (BLI) administered via intravenous infusion. Per the applicant, RECARBRIO™ is indicated for the treatment of hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by susceptible Gram-negative bacteria. RECARBRIO™ is also indicated for complicated urinary tract infections (cUTI) and complicated intra-abdominal infections (cIAI) and was approved for new technology add-on payment for these indications in the FY 2021 IPPS/LTCH PPS final rule (85 FR 58728). The maximum payment a hospital can receive for this is $9,577 as a new technology add on payment FY2022. Approved as new as this was for new diagnosis treatment for pneumonias and not the UTI on previous listed above.
- Codes to report:
XW033U5 – Introduction of Imipenem-cilastatin-relebactam Anti-infective into peripheral vein, percutaneous, new technology group 5 or
XW043U5 – Introduction of Imipenem-cilastatin-relebactam Anti-infective into central vein, percutaneous, new technology group 5
CMS received 26 applications for new technology add-on payments for FY 2022 under this traditional new technology add-on payment pathway. Only 16 were considered.
NOT APPROVED:
Aidoc Briefcase for PE™ Code XXE3X27 (Measurement of pulmonary artery flow, computer-aided triage and notification, new technology group 7).
Breyanzi® (lisocabtagene maraleucel) Codes XW03/43N7 – Introduction of Lisocabtagene Maraleucel Immunotherapy, Autologous into peripheral or central vein, percutaneous, new technology group 7
Ellipsys® Vascular Access System Codes X2KB317 and X2KC317 (Bypass right/left radial artery using thermal resistance energy, percutaneous approach, new technology group 7).
ENSPRYNG™ (satralizumab-mwge) Code XW01397- Introduction of Satrakuzynab-nwge percutaneously into subcutaneous tissue New Technology 7
INDIGO® Aspiration System with Lightning Tubing (“INDIGO® with Lightning”) Codes X2C-3T7 – Extirpation of upper or lower vein or artery, right or left, abd aorta, great vessel, percutaneous, Computer-aided Mechanical Aspiration, new technology group 7
Olumiant® (baricitinib) Codes could be 3E0DXGC, 3E0G7GC, XW0DXF5, 3E0H7GC, XW0DXM6, XW0G7M6, XW0H7M6 (Did not receive FDA approval by 7/1/21. This will receive NCTAP for Covid payments through FY2022 but not for new technology.
Pure-Vu® System Code XDPH8K7 (Irrigation of lower GI using intraoperative single-use oversleeve, via natural or artificial opening endoscopic, new technology group 7).
Rapid ASPECTS Code XXE0X07 (Measurement of intracranial vascular activity, computer-aided assessment, new technology group 7).
Steripath® MicroTM Blood Collection System (Steripath® MicroTM ISDD® ) (“Steripath Micro”) Code XXE5XR7- Measurement, Circulatory, External, Infection, Mechanical Initial specimen Diversion Technique Using Active Negative Pressure, New Technology 7.
New COVID-19 Treatments Add-On Payment (NCTAP)
cms.gov/medicare/covid-19/new-covid-19-treatments-add-payment-nctap
Codes for Remdesivir or COVID-19 Convalescent Plasma for Hospital Discharges on or after November 2, 2020
| ICD-10-PCS Code | Description |
|---|---|
| XW033E5 | Introduction of remdesivir anti-infective into peripheral vein, percutaneous approach, new technology group 5 |
| XW043E5 | Introduction of remdesivir anti-infective into central vein, percutaneous approach, new technology group 5 |
| XW13325 | Transfusion of convalescent plasma (nonautologous) into peripheral vein, percutaneous approach, new technology group 5 |
| XW14325 | Transfusion of convalescent plasma (nonautologous) into central vein, percutaneous approach, new technology group 5 |
Codes for Baricitinib for Hospital Discharges between November 19, 2020 and December 31, 2020*
| ICD-10-PCS Code | Description |
|---|---|
| XW0DXF5 | Introduction of other new technology therapeutic substance into mouth and pharynx, external approach, new technology group 5 |
| 3E0G7GC | Introduction of other therapeutic substance into upper G.I. via natural or artificial opening |
| 3E0H7GC | Introduction of other therapeutic substance into lower G.I. via natural or artificial opening |
*In accordance with the EUA, providers should administer baricitinib with remdesivir. Claims should also include the code for remdesivir (XW033E5 or XW043E5).
Codes for Baricitinib for Hospital Discharges on or after January 01, 2021 through the End of the COVID-19 PHE*
ICD-10-PCS Code Description
| XW0DXM6 | Introduction of baricitinib into mouth and pharynx, external approach, new technology group 6 |
| XW0G7M6 | Introduction of baricitinib into upper GI, via natural or artificial opening, new technology group 6 |
| XW0H7M6 | Introduction of baricitinib into lower GI, via natural or artificial opening, new technology group 6 |
*In accordance with the EUA, providers should administer baricitinib with remdesivir. Claims should also include the code for remdesivir (XW033E5 or XW043E5).
Hospitals should report the ICD-10-PCS code(s) for all products administered during the stay, even if the hospital got the product for free. Hospitals shouldn’t report charges for products they got for free.
| Note: |
|---|
| A hospital shouldn’t seek additional payment on the claim for drugs or biologicals to treat patients with known or suspected COVID-19 that the government purchased or provided for free. See the CMS Medicare Claims Processing Manual, Pub. 100-04, Chapter 32, Section 67 (PDF). |
For more information on COVID-19 diagnosis and procedure codes, visit the “Latest News” section of the MS-DRG Classifications and Software webpage.
You can also review our COVID-19 FAQs (PDF), which include information on NCTAP and our implementation of Section 3710 of the CARES Act.
The information contained in this post is valid at the time of posting. Viewers are encouraged to research subsequent official guidance in the areas associated with the topic as they can change rapidly.





